-
No results without a charge...
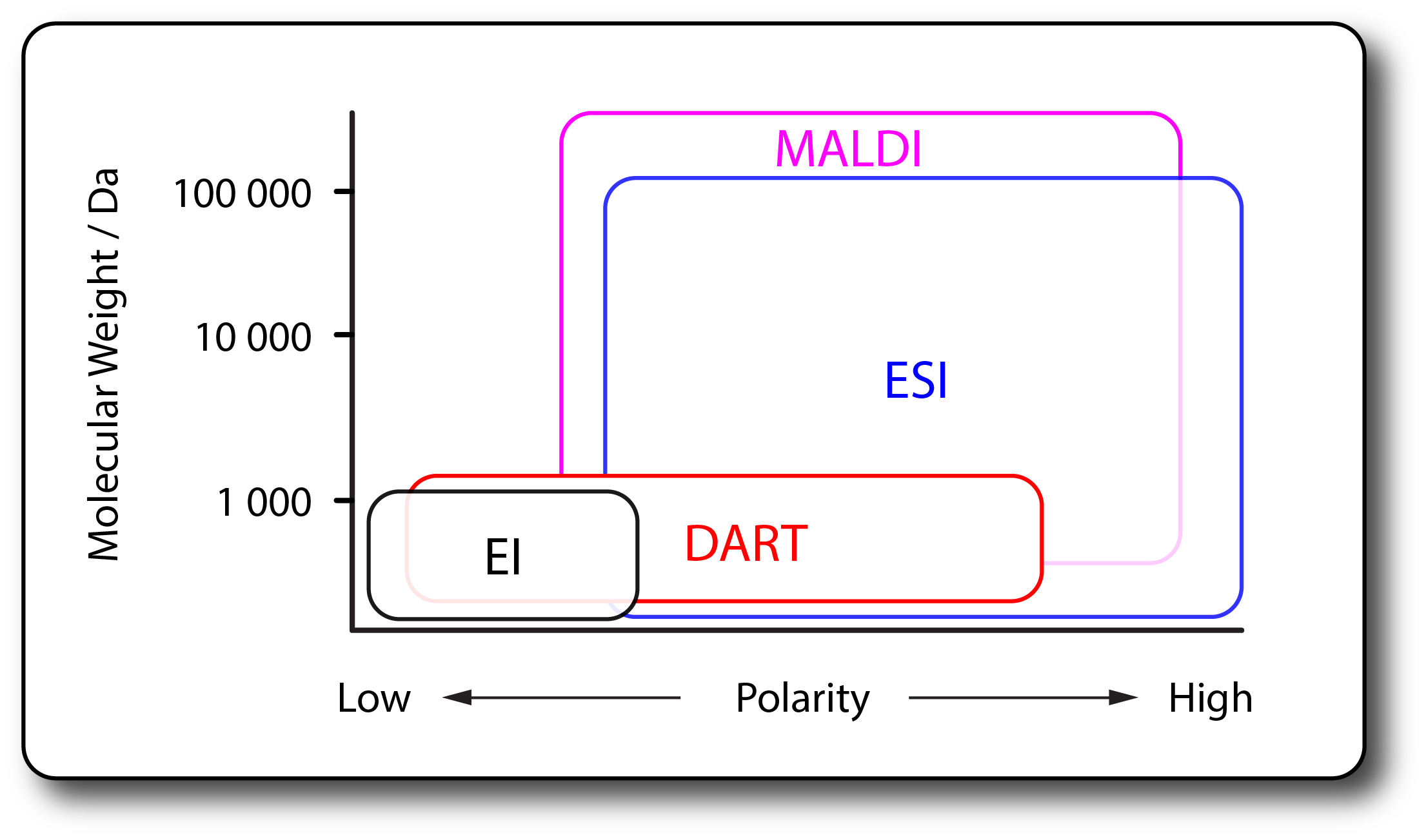
- All mass spectrometers use electric and/or magnetic fields to separate an ensemble of ions on the basis of their mass-to-charge ratio (m/z).
- All MS analyses therefore require that stable, gas-phase ions be produced from neutral, condensed phase analytes.
- Numerous ionization techniques have been invented, some of which have transformed the field of mass spectrometry.
- Explore the tabs to learn about the ion sources available in AIMS.
-
Electrospray Ionization
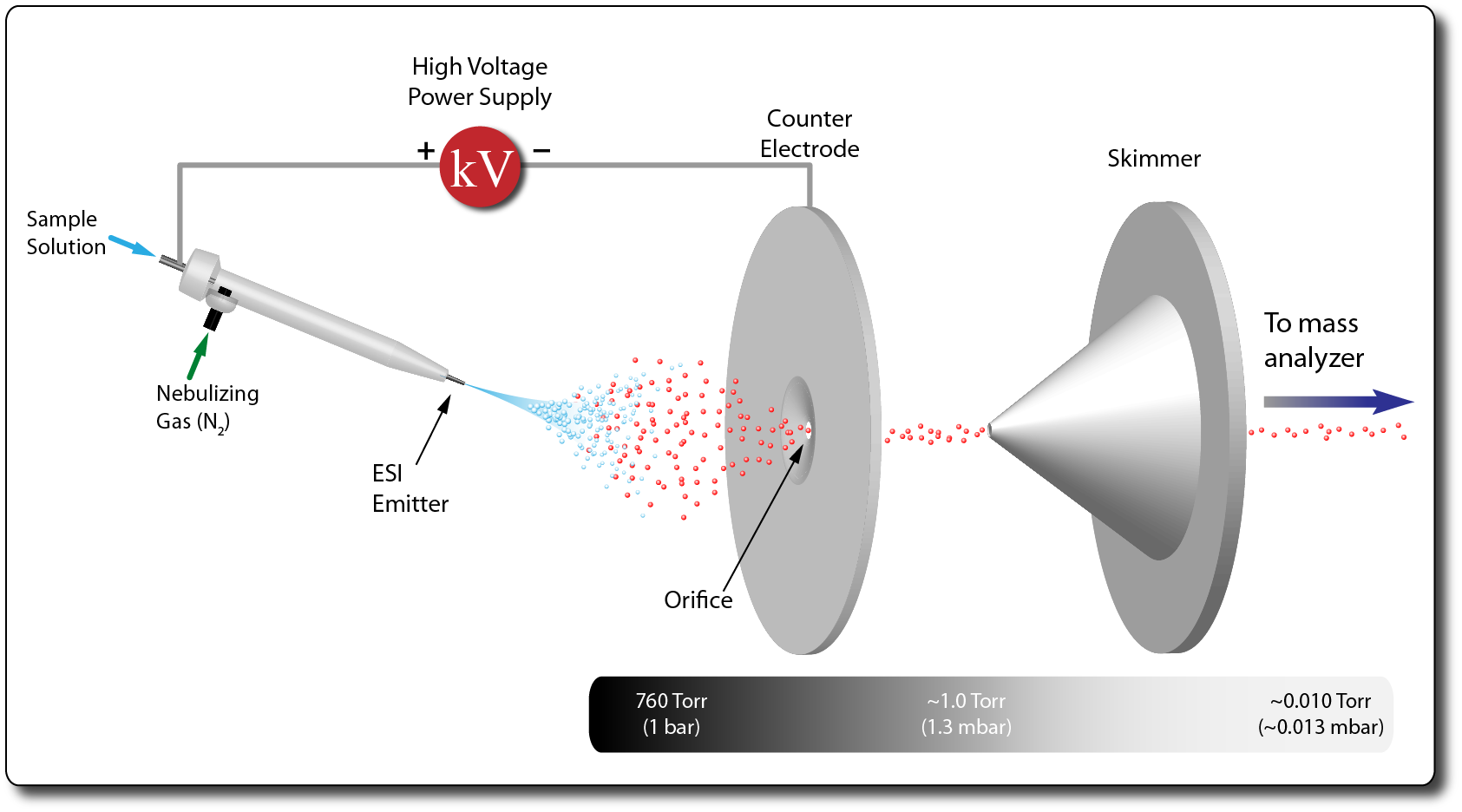
- Electrospray ionization (ESI) is a method to generate gas-phase ions from solution-phase analytes at atmospheric pressure.
- Compounds are dissolved in mixtures of polar solvents such as water, methanol or acetonitrile and often contain volatile organic modifiers such as formic acid or ammonium acetate.
- The liquid solution flows through a fine capillary (the emitter) to which a high voltage electric potential (~1 to 5 kV) is applied.
- The intense electric field at the emitter induces formation of a Taylor cone yielding charged droplets which begin to evaporate solvent and shrink until gaseous ions remain or are released.
- ESI is considered a ‘soft’ ionization mechanism characterized by formation of intact, even-electron ion species often forming [M+H]+ or [M-H]- ion species.
- Other charging adducts are also common (e.g. NH4+, Na+, K+, HCOO-, etc.)
- ESI is suitable for a wide range of compounds including small organic molecules, peptides, proteins, oligonucleotides, oligosaccharides, organometallic complexes, synthetic polymers and more.
References- Fenn, J. B. Angew. Chem., Int. Ed. 2003, 42, 3871. https://doi.org/10.1002/anie.200300605
- Kebarle, P.; Verkerk, U. H. Mass Spectrom. Rev. 2009, 28, 898. https://doi.org/10.1002/mas.20247
- Konermann, L. et al; Anal. Chem. 2013, 85, 1, 2-9 https://doi.org/10.1021/ac302789c
-
DART
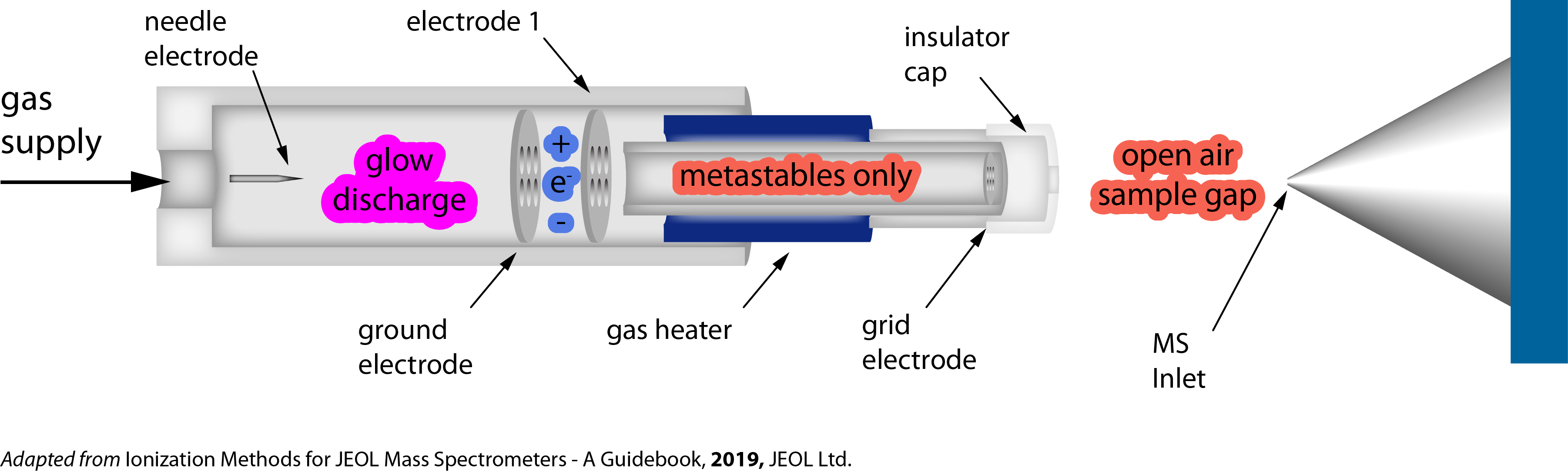
- Direct Analysis in Real Time (DART) ionization is an ambient ionization source that enables immediate analysis of (typically) small, moderately polar compounds in the open atmosphere with little or no sample preparation.
- A gas (usually He) flows through a chamber within which a glow discharge produces ions and excited-state atoms.
- The gas is heated to a temperature up to 500°C and exits the DART source through a grid electrode and ceramic insulators.
- Samples placed in the DART stream are first vapourized, then ionized and swept into the inlet of the mass spectrometer for analysis.
- Commonly observed ion species are [M+H]+ and [M+NH4]+ in positive polarity and [M-H]- in negative polarity.
References- Cody, R.B., et al.; Anal. Chem. 2005, 77, 2297-2302 https://doi.org/10.1021/ac050162j
- Cody, R.B., et al.; JEOL News 2005, 40, 8-12. Direct Analysis in Real Time (DART®) Mass Spectrometry
- Ionization Methods for JEOL Mass Spectrometers – A Guidebook; JEOL Ltd. 2019
- DART Applications Notebook 2016; JEOL Ltd. 2016
-
Electron Ionization
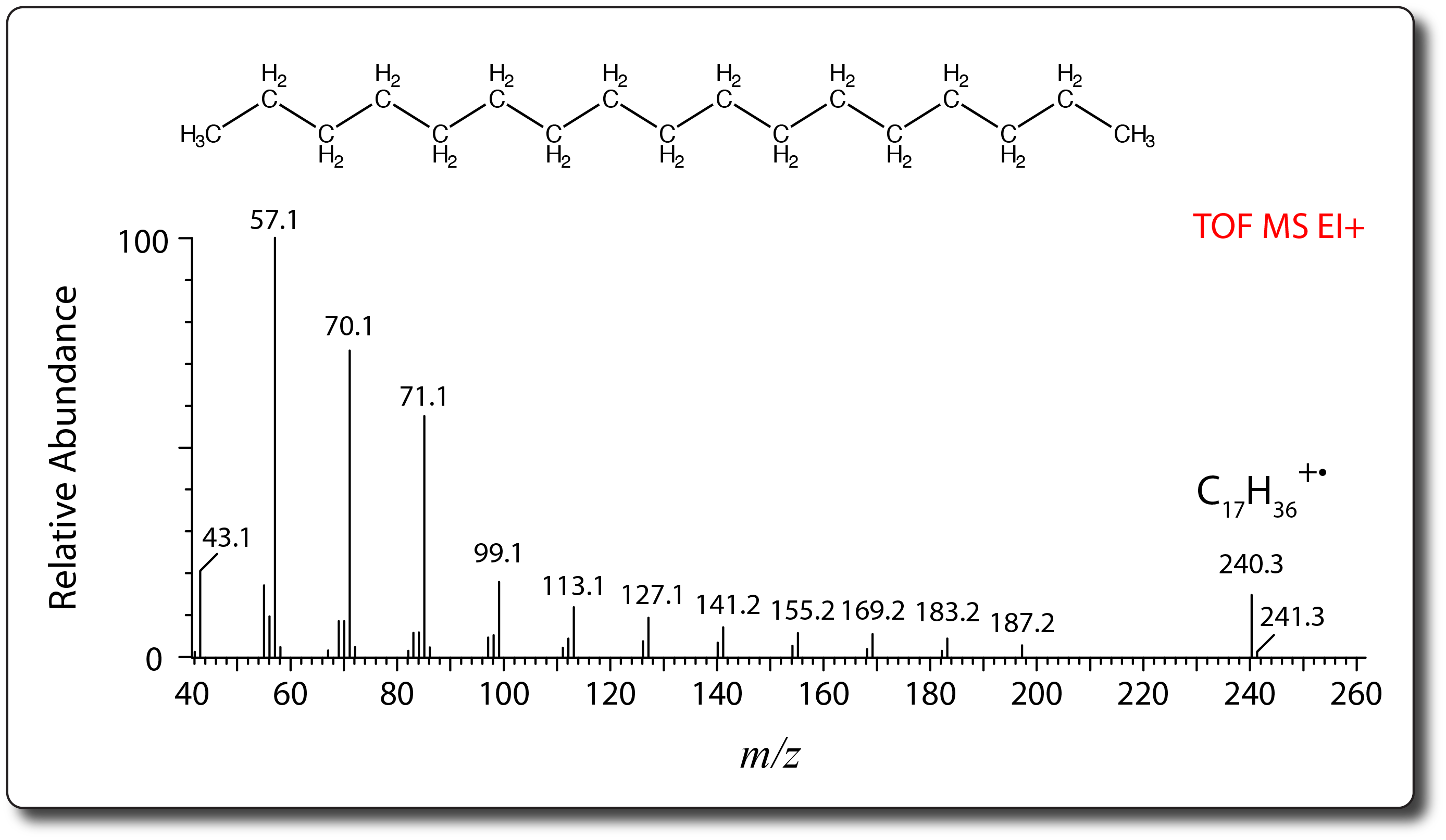
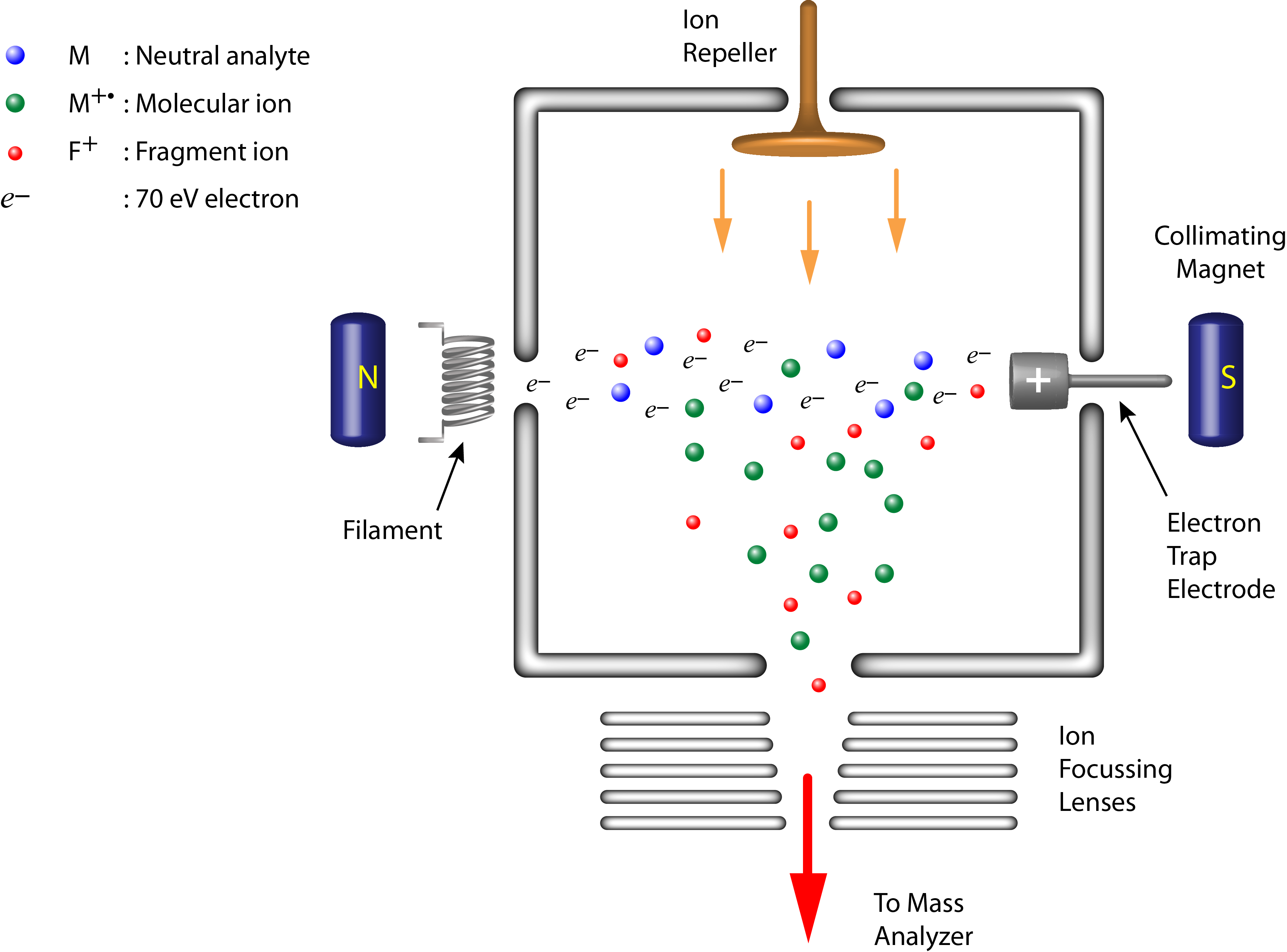
- Electron Ionization (EI) is one of the earliest and most commonl ionization sources used in mass spectrometry.
- It is a vacuum ionization technique by which gaseous samples are ionized in a stream of energetic electrons (usually 70 eV) emitted from a filament.
- Typically applied for small, non-polar, organic molecules, EI is well suited to be coupled with gas chromatography (GC).
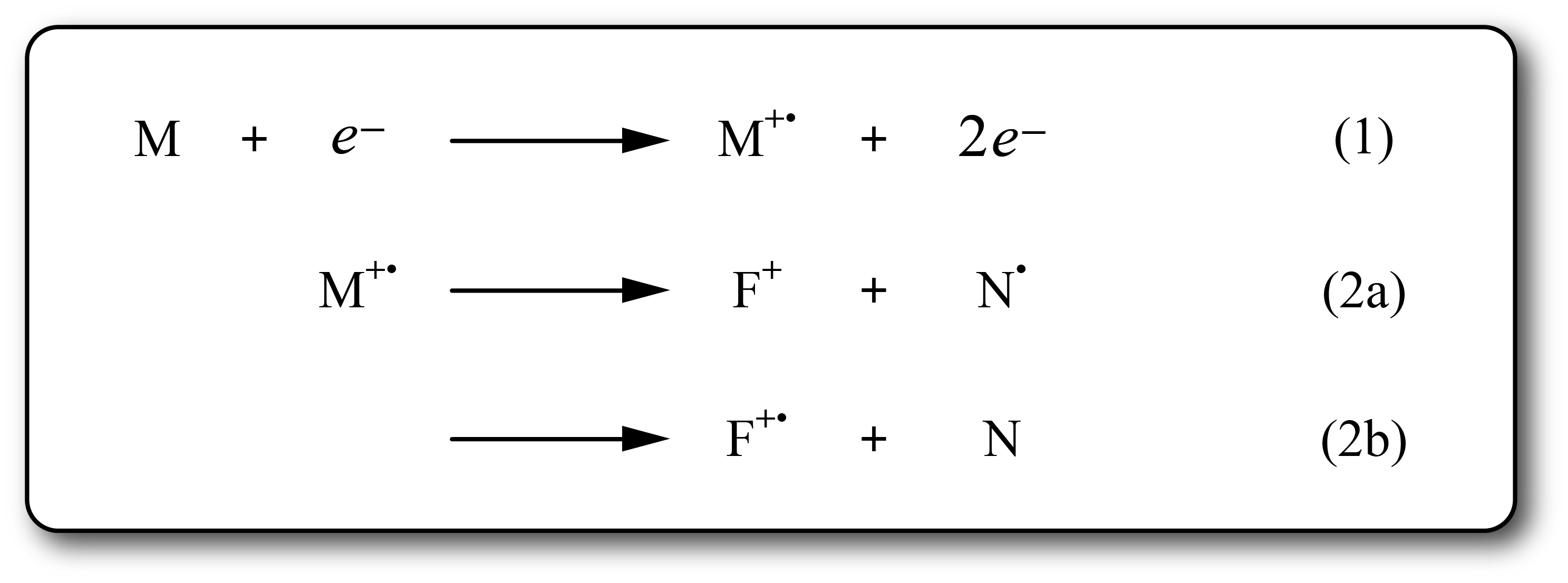
- EI is considered a ‘hard’ ionization technique and spectra are often complex showing unique and structurally-relevant fragment ions in addition to the characteristic molecular ion (M+·).
- EI spectra are therefore suitable for compound identifcation through spectral library searching.
EI Ion Source Schematic
Equations
-
MALDI
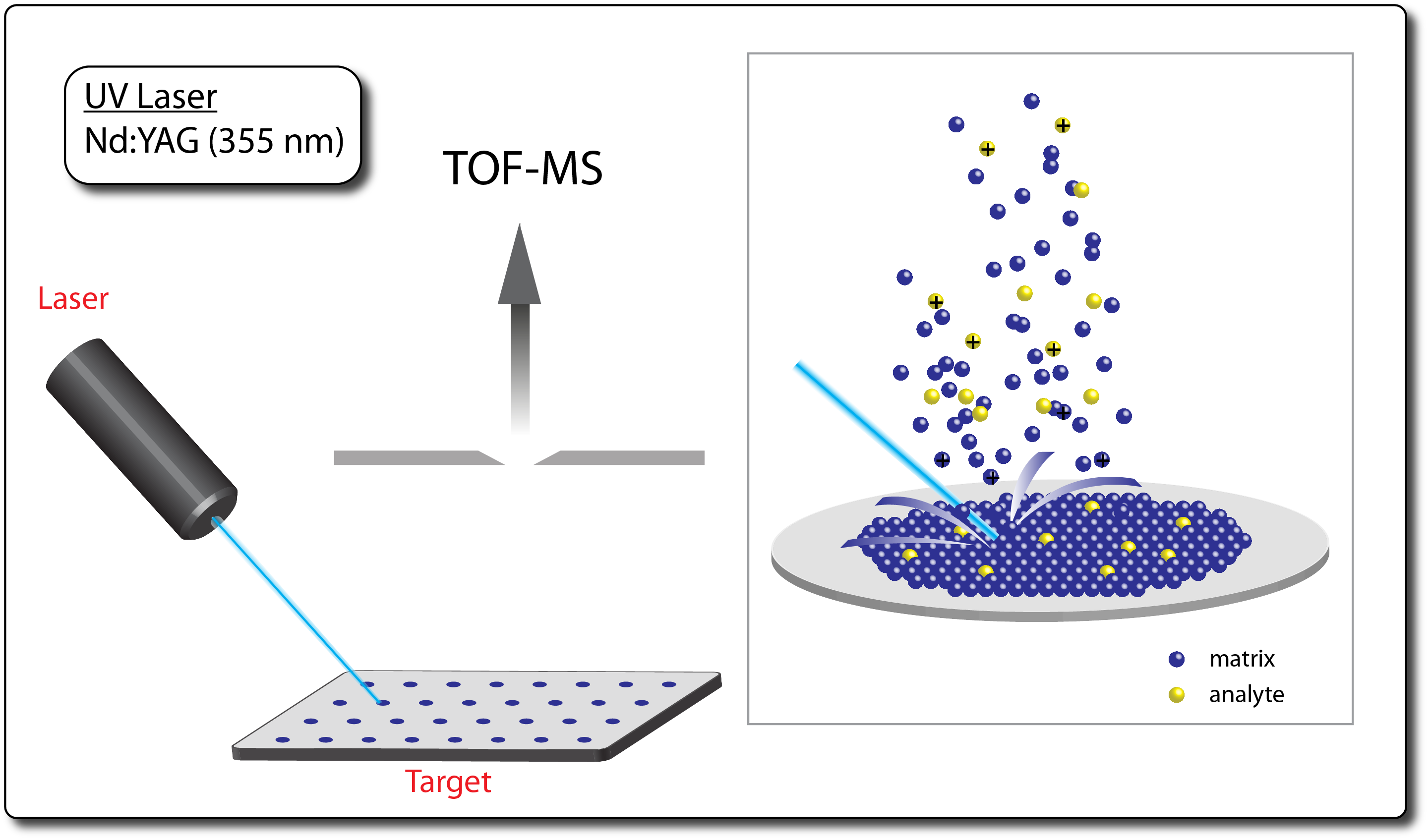
- Matrix-assisted laser desorption / ionization (MALDI) is an ionization technique that uses a laser to generate ions from analyte molecules co-crystallized in a solid matrix.
- The matrix is typically a small aromatic compound having a high absorption crosssection at the wavelength of the laser, usually in the UV region.
- MALDI is a 'soft' ionization technique and is often considered complimentary to ESI; however, whereas ESI is known for production of multiply-charged ions, MALDI often produces predominantly lower charge states (e.g. +1 or +2).
- Typical applications include biopolymers (e.g. proteins, peptides, carbohydrates, oligonucleotides, etc.) and large organic compounds (e.g. synthetic polymers, dendrimers, etc.).
 References
References
- Introduction to MALDI-TOF-TOF, Bruker Daltonics Ltd. 2016
- Ionization Methods for JEOL Mass Spectrometers – A Guidebook; JEOL Ltd. 2019
